Titanium dioxide nanoreactor
The titanium dioxide nanoparticles crystallize in a polymer network at room temperature.
Tiny particles of titanium dioxide are found as key ingredients in wall paints, sunscreens, and toothpaste; they act as reflectors of light or as abrasives. However with decreasing particle size and a corresponding change in their surface-to-volume ratio, their properties change so that crystalline titanium dioxide nanoparticles acquire catalytic ability: Activated by the UV component in sunlight, they break down toxins or catalyze other relevant reactions.
Now, Dr. Katja Henzler and a team of chemists at the Helmholtz Centre Berlin have developed a synthesis to produce nanoparticles at room temperature in a polymer network. Their analysis, conducted at BESSY II, Berlin's synchrotron radiation source, has revealed the crystalline structure of the nanoparticles. This represents a major step forward in the usage of polymeric nanoreactors since, until recently, the nanoparticles had to be thoroughly heated to get them to crystallize. The last synthesis step can be spared due to the special environment inside the PNIPAM network.
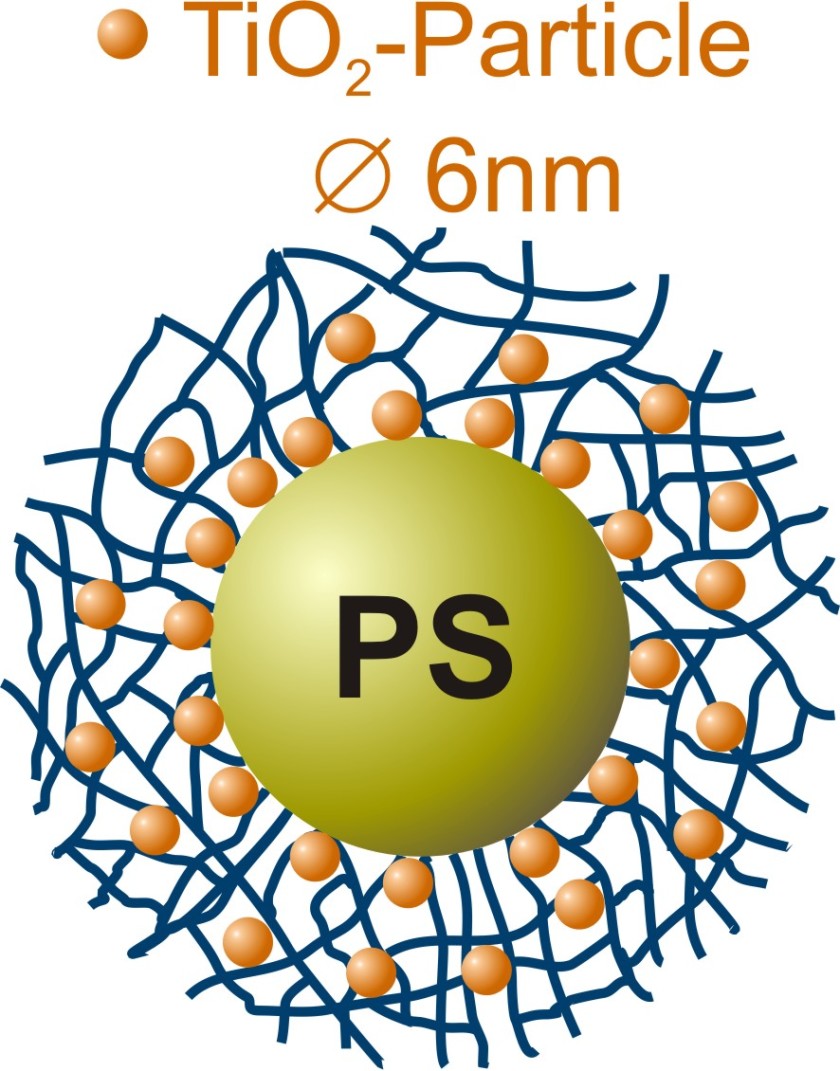
The Henzler team's polymeric nanoreactors consist of a polystyrene core surrounded by a network of PNIPAM chains. A titanium compound was added to an ethanolic solution of the polymer colloids, which did trigger the formation of small titanium dioxide particles within the PNIPAM network. The BESSY II experiments showed that the chemists were able to control the speed of these processes while at the same time affecting the quality of the nanocrystals that had formed.
Using the novel combination of x-ray microscopy and spectroscopy (NEXAFS-TXM, U41-SGM) at BESSY II, Henzler and the microscopy team were able to show that the nanoparticles are homogeneously distributed over the polymeric nanoreactors. The researchers examined their samples in a cryogenic aqueous environment, which prevents artifact formation due to sample drying. Their analysis showed that the nanoparticles have a crystalline structure. "The nanocrystals have a tetragonal anatase structure and this crystalline structure is a key to their catalytic performance. Additionally, our new analytic method allows us to control the quality of the synthesized particles so that we can optimize them for relevant applications," says Katja Henzler.
Nano Letters, 2013, 13 (2), pp 824–828;
DOI: 10.1021/nl3046798
https://www.helmholtz-berlin.de/pubbin/news_seite?nid=13668;sprache=en
- Copy link
-
Battery research with the HZB X-ray microscope
New cathode materials are being developed to further increase the capacity of lithium batteries. Multilayer lithium-rich transition metal oxides (LRTMOs) offer particularly high energy density. However, their capacity decreases with each charging cycle due to structural and chemical changes. Using X-ray methods at BESSY II, teams from several Chinese research institutions have now investigated these changes for the first time with highest precision: at the unique X-ray microscope, they were able to observe morphological and structural developments on the nanometre scale and also clarify chemical changes.
-
BESSY II: New procedure for better thermoplastics
Bio-based thermoplastics are produced from renewable organic materials and can be recycled after use. Their resilience can be improved by blending bio-based thermoplastics with other thermoplastics. However, the interface between the materials in these blends sometimes requires enhancement to achieve optimal properties. A team from the Eindhoven University of Technology in the Netherlands has now investigated at BESSY II how a new process enables thermoplastic blends with a high interfacial strength to be made from two base materials: Images taken at the new nano station of the IRIS beamline showed that nanocrystalline layers form during the process, which increase material performance.
-
Hydrogen: Breakthrough in alkaline membrane electrolysers
A team from the Technical University of Berlin, HZB, IMTEK (University of Freiburg) and Siemens Energy has developed a highly efficient alkaline membrane electrolyser that approaches the performance of established PEM electrolysers. What makes this achievement remarkable is the use of inexpensive nickel compounds for the anode catalyst, replacing costly and rare iridium. At BESSY II, the team was able to elucidate the catalytic processes in detail using operando measurements, and a theory team (USA, Singapore) provided a consistent molecular description. In Freiburg, prototype cells were built using a new coating process and tested in operation. The results have been published in the prestigious journal Nature Catalysis.
